Chim., 1979, 10, 763-772. WebEnthalpy of formation ( Hf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from Chem. . been selected on the basis of sound scientific judgment. [all data], Go To: Top, Condensed phase thermochemistry data, References. Eng. Heats of combustion and formation of the paraffin hydrocarbons at 25 C, Good, W.D. 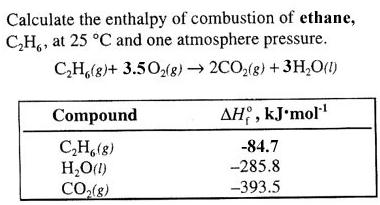 Chem. What is the standard enthalpy of formation of CoH14, given the standard enthalpies of formation of CO2 (g) and H2O (l) are -394 and -286 kJ/mol, respectively? Isobaric heat capacities at bubble point. J. Example \(\PageIndex{1}\): Enthalpy of Formation. Thermodyn., 1983, 15, 1087-1092. (U.S.), 1945, 35, 3, 219-244, https://doi.org/10.6028/jres.035.009 Webstandard enthalpy of formation of hexanecheese trail wisconsin lodging. Acad. [all data], Watanabe, Nakayama, et al., 1962 WebNow do the calculation: Hess's Law says that the enthalpy changes on the two routes are the same. Heat capacities of liquids at temperatures between 90 and 300 K and at atmospheric pressure. Ultrasonic speeds and isentropic compressibilities of 2-methylpentan-1-ol with hexane isomers at 298.15 K, All standard enthalpies have the unit kJ/mol. J. ; Uncertainty assigned by TRC = 0.007 l/mol; Based on data from 286. Experimental study of isobaric specific heat of higher alcohols at high pressures, [all data], Letcher and Marsicano, 1974 shall not be liable for any damage that may result from It is not possible to measure the value of \(H^oo_f\) for glucose, 1273.3 kJ/mol, by simply mixing appropriate amounts of graphite, \(\ce{O2}\), and \(\ce{H2}\) and measuring the heat evolved as glucose is formed sincethe reaction shown in Equation \(\ref{7.8.2}\) does not occur at a measurable rate under any known conditions. 1, 1988, 84(11), 3991-4012. https://en.wikipedia.org/w/index.php?title=Hexane_(data_page)&oldid=1128617892, Creative Commons Attribution-ShareAlike License 3.0, Except where noted otherwise, data relate to, This page was last edited on 21 December 2022, at 02:16. J. Chem. [all data], Parks, Huffman, et al., 1930 I. The standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and products using Hess's law. Consequently, the enthalpy changes (from Table T1) are, \[ \begin{matrix} \Delta H_{3}^{o} = \Delta H_{f}^{o} \left [ CO_{2} \left ( g \right ) \right ] = 6 \; \cancel{mol \; CO_{2}}\left ( \dfrac{393.5 \; kJ}{1 \; \cancel{mol \; CO_{2}}} \right ) = -2361.0 \; kJ \\ \Delta H_{4}^{o} = 6 \Delta H_{f}^{o} \left [ H_{2}O \left ( l \right ) \right ] = 6 \; \cancel{mol \; H_{2}O}\left ( \dfrac{-285.8 \; kJ}{1 \; \cancel{mol \; H_{2}O}} \right ) = -1714.8 \; kJ \end{matrix} \]. Example #8: Using standard enthalpies of formation, calculate the heat of combustion per mole of gaseous water formed during the complete combustion of ethane gas. Eng. Show Sources Licenses and Attributions Previous Next (c=4.18 Jg-1K-1). Not in this one. The handling of this chemical may incur notable safety precautions. Faraday Trans. Chem., 1975, 79, 574-577. LL - Sharon G. Lias and Joel F. Liebman Tardajos, G.; Aicart, E.; Costas, M.; Patterson, D., It does not use the full chemical equations and it is usually presented like this: Here's another to write this form of Hess' Law, one that slightly varies from the above manner: The "rxn" above is a common way to abbreviate "reaction." An. Am. To three sig figs, the value is 248 kJ/mol. ; Ausloos, P., [all data], Boublik, Fried, et al., 1984 in these sites and their terms of usage. WebThe chemical energy involved in a reaction is also called the enthalpy. Answer of Calculate the standard enthalpy of formation of hexane 6C (s) + 7H2 (g) ---> C6H14 (l) Thermodynam., 1991, 23, 247-259. WebThe boling point 36C/97F, and the vapors are heavier than air. Chem. Extrapolation below 91 K, 54.68 J/mol*K.; Extrapolation below 90 K, 64.02 J/mol*K.; Extrapolation below 90 K, 65.44 J/mol*K.; T = 308.35, 333.15. p = 0.1 MPa. Compare this value with the value calculated in Equation \(\ref{7.8.8}\) for the combustion of glucose to determine which is the better fuel. Pitzer K.S., 1.118 of the Thermochemical Network (2015); available at ATcT.anl.gov. [all data], Zawisza, 1985 [all data], Benson and D'Arcy, 1986 [all data], Naziev, Bashirov, et al., 1986 Am. [all data], Michou-Saucet, Jose, et al., 1984 Bondi, A., Steiner, B.; Giese, C.F. The standard state of an element can be identified in Table T1: by a \(H^o_f\) value of 0 kJ/mol. 2. Rogers, D.W.; Crooks, E.L., [ 4 ], and was also used for the initial development of high-accuracy ANLn composite electronic structure methods [ 5 ]. Chem., 1951, 43, 946-950. Heats of combustion and formation of the paraffin hydrocarbons at 25 C, Values of \(H^o_f\) for an extensive list of compounds are given in Table T1. Waddington, Guy; Douslin, Donald R., Catal., 1984, 9, 219-223. Note how the enthalpy of formation for hexane (the desired result) is our only unknown. Thermodynam., 1984, 16, 73-79. Also, we need to have the equation balanced, so be sure to remember to check for that. Am. I. Esters of unsaturated fatty acids, The Journal of Chemical Thermodynamics, 1985, 17, 12, 1171-1186, https://doi.org/10.1016/0021-9614(85)90044-8 GT - Glushko Thermocenter, Russian Academy of Sciences, Moscow, Go To: Top, Gas phase thermochemistry data, Phase change data, Reaction thermochemistry data, Henry's Law data, Gas phase ion energetics data, References, Notes, Data compiled as indicated in comments: 1. uses its best efforts to deliver a high quality copy of the Soc., 1936, 58, 146-153. WebQuestion: b) The standard enthalpy of formation of hexane (C6H14) can be determined under standard conditions using standard enthalpy of combustion data for its constituent elements. Zhur., 1986, 51, 998-1004. [all data], Ohnishi, Fujihara, et al., 1989 also available. Chem. The sign convention is the same for all enthalpy changes: negative if heat is released by the system and positive if heat is absorbed by the system. [all data], Ikuta, Yoshihara, et al., 1973 [all data], Williamham, Taylor, et al., 1945 Chem. Eng. Uchebn. Data compiled as indicated in comments: d(ln(kH))/d(1/T) = Temperature dependence constant (K), Go To: Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Phase change data, Reaction thermochemistry data, Henry's Law data, References, Notes, Data evaluated as indicated in comments: [Total 3 marks] 7. ; Marsicano, F., Soc., 1931, 53, 3876-3888. [all data], Connolly, Sage, et al., 1951 dermatology brevard county Facebook-f leed's certified refill 9092 03rf Twitter effect of budget deficit on economic growth Instagram seventy five pronunciation Linkedin. [all data], Roth, Kirmse, et al., 1982 Thermodynam., 1980, 12, 891-896. For example, although oxygen can exist as ozone (O3), atomic oxygen (O), and molecular oxygen (O2), O2 is the most stable form at 1 atm pressure and 25C. Kinet., 1976, 8, 725. following TRC products: Go To: Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Phase change data, Henry's Law data, Gas phase ion energetics data, References, Notes, Data compiled as indicated in comments: Note that while the majority of the values of standard enthalpies of formation are exothermic, or negative, there are a few compounds such as NO(g) and N2O4(g) that actually require energy from its surroundings during its formation; these endothermic compounds are generally unstable. J. Chem. We must therefore multiply this value by the molar mass of tetraethyl lead (323.44 g/mol) to get \(H^o_{comb}\) for 1 mol of tetraethyl lead: \[\begin{align*}\Delta H_{comb}^{o} &= \left ( \dfrac{-19.29 \; kJ}{\cancel{g}} \right )\left ( \dfrac{323.44 \; \cancel{g}}{mol} \right ) \\[4pt] &= -6329 \; kJ/mol \end{align*}\], Because the balanced chemical equation contains 2 mol of tetraethyllead, \(H^o_{rxn}\) is, \[\begin{align*}\Delta H_{rxn}^{o} &= 2 \; \cancel{mol \; \left ( C_{2}H{5}\right )_4 Pb} \left ( \dfrac{-6329 \; kJ}{1 \; \cancel{mol \; \left ( C_{2}H{5}\right )_4 Pb }} \right ) \\[4pt] &= -12,480 \; kJ \end{align*}\], C Inserting the appropriate values into the equation for \(H^o_f [\ce{(C2H5)4Pb}]\) gives, \[ \begin{align*} \Delta H_{f}^{o} \left [ \left (C_{2}H_{4} \right )_{4}Pb \right ] & =\left [1 \; mol \;PbO \;\times 219.0 \;kJ/mol \right ]+\left [8 \; mol \;CO_{2} \times \left (-393.5 \; kJ/mol \right )\right ]+\left [10 \; mol \; H_{2}O \times \left ( -285.8 \; kJ/mol \right )\right ] + \left [-27/2 \; mol \; O_{2}) \times 0 \; kJ/mol \; O_{2}\right ] \left [12,480.2 \; kJ/mol \; \left ( C_{2}H_{5} \right )_{4}Pb \right ]\\[4pt] Its use was completely phased out in 1986 because of the health risks associated with chronic lead exposure. Soc., 1946, 68, 1704-1708. The value of \(H^o_{rxn}\) is -179.4 kJ/mole\(\ce{H2SO4}\). WebThe standard enthalpy of combustion of liquid hexane (C6H14) is -4163 kJ/mole. Thermal data on organic compounds. NIST Standard Reference This work was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences and Biosciences under Contract No. where the symbol \(\sum\) means sum of and \(m\) and \(n\) are the stoichiometric coefficients of each of the products and the reactants, respectively. J. Chem. Soc., Thermodynamic properties of decalins mixed with hexane isomers at 298.15K. The formation of any chemical can be as a reaction from the corresponding elements: elements compound which in terms of the the Enthalpy of ; Mautner(Meot-Ner), M., Connolly, T.J.; Sage, B.H. On your diagram label the enthalpy change of reaction, H, and the activation energy, Ea. Vyssh. Turner, R.B. J. Chem. S. J. Klippenstein, L. B. Harding, and B. Ruscic. Die Berechnung von Resonanzenergien; das MM2ERW-Kraftfeld, Prosen, E.J.R. The standard enthalpy of complete combustion of liquid hexane (C6H14) is -4163 kJ/mol. Am. Chem. Rev., 1946, 39, 435-447. Enthalpies of combustion of toluene, benzene, cyclohexane, cyclohexene, methylcyclopentane, 1-methylcyclopentene, and n-hexane, Estimated ionization potentials, J. The purpose of the fee is to recover costs associated An. 1) Let us assume that the carbon is in its standard state of graphite (as opposed to diamond or buckminsterfullerene). [all data], Czarnota, 1979 MS - Jos A. Martinho Simes. ; Benson, G.C., The elemental form of each atom is that with the lowest enthalpy in the standard state. Thermodynam., 1982, 14, 303-308. The standard state for measuring and reporting enthalpies of formation or reaction is 25. Grolier, J.P.E. Molar excess volumes and excess heat capacities of (1,2,4-trichlorobenzene + an alkane), [all data], Waddington and Douslin, 1947 Data, 1963, 8, 3, 371-381, https://doi.org/10.1021/je60018a027 Neft Gaz 18, 1975, No.10, 63-66. Experimental study of isobaric specific heat of higher alcohols at high pressures, Effect of fluoro substituents on the thermal rearrangement of cyclopropane systems, on behalf of the United States of America. I 1 2:05 PM 12/10/2020 (2 Show transcribed image text Expert Answer 100% (1 rating) Given enough time, diamond will revert to graphite under these conditions. Excess volumes excess heat capacities of some mixtures: (an isomer of hexanol + an n-alkane) at 298.15 K, Enthalpy of formation (\(H_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. (1) ii) Hexane melts at . Fluid Phase Equilib., 1989, 46, 59-72. ; Halpin, C.J. [all data], Perez-Casas, Aicart, et al., 1988 ; Sugamori, M.E., Mautner(Meot-Ner), M.; Sieck, L.W. Tetraethyllead is a highly poisonous, colorless liquid that burns in air to give an orange flame with a green halo. An. Sci. Germain Henri Hess, in 1840, discovered a very useful principle which is named for him: There is another way to use Hess' Law. K. See also, Based on data from 300. Carbon naturally exists as graphite and diamond. [all data], Molnar, Rachford, et al., 1984 [all data], Czarnota, 1979 L - Sharon G. Lias, Data compiled as indicated in comments: 4) The above equations, when added, will produce the formation equation for methyl bromide. Given: reactant, products, and \(H^_{comb}\) values. houston area women's center clothing donations; hobbies for adults with adhd; hillside memorial park find a grave; badlands without sasquatch; farmington mo obituaries; this is gonna hurt isn t it meme girl; liberty grace lawrence; hart house restaurant kevin hart J. Photoelectron spectra of molecules. Ann. Proc. Sci., 1939, A9, 109-120. The enthalpy change for the formation of 1 mol of a compound from its component elements when the component elements are each in their standard states. Sci., 1939, A9, 109-120. J. Chem. Example #6: Ammonia reacts with oxygen to form nitrogen dioxide and steam, as follows: Given the following standard enthalpies of formation (given in kJ/mol), calculate the enthalpy of the above reaction: Note that water is given as a gas. Aicart, E.; Kumaran, M.K. WebEnthalpy (Crystal 1 in equilibrium with Gas) as a function of Temperature Temperature from 0.0002 K to 160.023 K Enthalpy (Liquid in equilibrium with Gas) as a function of Temperature Temperature from 177.878 K to 497.38 K Enthalpy (Ideal Gas) as a function of Temperature Temperature from 200 K to 1500 K Entropy [all data], Stephenson and Malanowski, 1987 Inserting these values into Equation \(\ref{7.8.7}\) and changing the subscript to indicate that this is a combustion reaction, we obtain, \[ \begin{align} \Delta H_{comb}^{o} &= \left [ 6\left ( -393.5 \; kJ/mol \right ) + 6 \left ( -285.8 \; kJ/mol \right ) \right ] - \left [-1273.3 + 6\left ( 0 \; kJ\;mol \right ) \right ]\label{7.8.8} \\[4pt] &= -2802.5 \; kJ/mol \end{align} \], As illustrated in Figure \(\PageIndex{2}\), we can use Equation \(\ref{7.8.8}\) to calculate \(H^_f\) for glucose because enthalpy is a state function. . ; Huffman, H.M.; Thomas, S.B., Molar excess volumes and excess heat capacities of (1,2,4-trichlorobenzene + an alkane), J. Chem. WebEnthalpy of formation ( Hf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. [all data], Costas and Patterson, 1985 [all data], Lias, Ausloos, et al., 1976 Ucheb. [all data], Tardajos, Aicart, et al., 1986 Consider the general reaction, \[ aA + bB \rightarrow cC + dD \label{7.8.3}\]. Benson, G.C. J. WebThe standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of pressure and 298.15 K) is formed from its pure elements under the same conditions. Excess volumes excess heat capacities of some mixtures: (an isomer of hexanol + an n-alkane) at 298.15 K, 7.4: Standard Enthalpy of Formation is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. [all data], von Reis, 1881 Database and to verify that the data contained therein have ; Smith, N.K., Liquid structure and second-order mixing functions for benzene, toluene, and p-xylene with n-alkanes, J. Chem. ; D'Arcy, P.J., Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Enthalpy of hydrogenation of the hexadienes and cis- and trans-1,3,5-hexatriene, ; Roth, W.R.; Schroder, G., Int. ; Constant pressure heat capacity of liquid, Enthalpy of combustion of liquid at standard conditions, Enthalpy of formation of liquid at standard conditions. 1) The enthalpy of combustion for hexane, carbon and hydrogen are these chemical equations: 2) To obtain the target reaction (the enthalpy of formation for hexane), we must do the following: By the way, the second equation (presented as the enthalpy of combustion of carbon) is also the equation for the formation of carbon dioxide. ; Lacey, W.N., ; Rossini, F.D., Br2(l) is the more stable form, which means it has the lower enthalpy; thus, Br2(l) has Hf = 0. J. Chem. Parks, G.S. Chem. Using the values in the above table of standard enthalpies of formation, calculate the Hreactiono for the formation of NO2(g). LBLHLM - Sharon G. Lias, John E. Bartmess, Joel F. Liebman, John L. Holmes, Rhoda D. Levin, and W. Gary Mallard [all data], Steiner, Giese, et al., 1961 B. Ruscic, R. E. Pinzon, G. von Laszewski, D. Kodeboyina, A. Burcat, D. Leahy, D. Montoya, and A. F. Wagner, B. Ruscic, Active Thermochemical Tables (ATcT) values based on ver. Ser. Neft i Gaz, 1984, (2), 60-62. Further studies on the heat capacities, entropies and free energies of hydrocarbons, [all data], Skinner and Snelson, 1959 Example #9: The H for the following reaction equals 89 kJ: In addition, these two standard enthalpies of formation are known: 2) Inserting values into the above, we find: 1) Here are all three data reactions written out in equation form: 2) What we need to do is add the three data equations together in such a way as to recover the target equation: 4) However, this is not the enthalpy of formation, since that value is always for one mole of the product. Dewar, M.J.S. Douslin, D.R. Chem. DH - Eugene S. Domalski and Elizabeth D. Hearing, Go To: Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Reaction thermochemistry data, Henry's Law data, Gas phase ion energetics data, References, Notes, Data compiled as indicated in comments: What is the standard enthalpy of formation (AHp) of liquid CoH14 given that the standard enthalpy Chem. Ann. Bonus Example: Given the following information: 1) The key is to see the meaning of 2LiOH(aq): 2) That means that, in reality, we want the H for this reaction: 5) Use Hess' Law utilizing the revised target equation: Hess' Law: two equations and their enthalpies, Hess' Law: three equations and their enthalpies, Hess' Law: four or more equations and their enthalpies. - 321. Web1) The enthalpy of combustion for hexane, carbon and hydrogen are these chemical equations: C6H14() + 192O2(g) ---> 6CO2(g) + 7H2O() C(s, gr) + O2(g) ---> CO2(g) H2(g) + 12O2(g) ---> H2O() 2) To obtain the target reaction (the enthalpy of formation for hexane), we must do the following: a) reverse the first equation Specific heat and related properties, What is the standard enthalpy of formation of tetraethyllead, given that \(H^_f\) is 19.29 kJ/g for the combustion of tetraethyllead and \(H^_f\) of red PbO(s) is 219.0 kJ/mol? [all data], Prosen and Rossini, 1941 Isobaric heat capacities at bubble point. Ind. NBS, 1945, 263-267. Data from NIST Standard Reference Database 69: The National Institute of Standards and Technology (NIST) n-Hexane, methylcyclopentane, and n-octane, ; Costas, M., i) State what is meant by standard conditions. Thermophysical properties of liquid n-hexane at temperatures from 243 K to 473 K and at pressures to 500 MPa, Parks, G.S. Trans. liquid phase; solvent: Glacial acetic acid; liquid phase; solvent: Acetic acid; Reanalyzed by, Constant pressure heat capacity of liquid, Temperature dependence parameter for Henry's Law constant, Enthalpy of combustion of liquid at standard conditions, Enthalpy of formation of gas at standard conditions, Enthalpy of formation of liquid at standard conditions, Enthalpy of reaction at standard conditions, Enthalpy of vaporization at standard conditions. Palmitic acid, the major fat in meat and dairy products, contains hydrogen, carbon, and oxygen, so the unbalanced chemical equation for its formation from the elements in their standard states is as follows: \[\ce{C(s, graphite) + H2(g) + O2(g) \rightarrow CH3(CH2)14CO2H(s)} \nonumber\], There are 16 carbon atoms and 32 hydrogen atoms in 1 mol of palmitic acid, so the balanced chemical equation is, \[\ce{16C (s, graphite) + 16 H2(g) + O2(g) -> CH3(CH2)14CO2H(s) } \nonumber\], \[ \ce{ Na (s) + 1/2 Cl2 (g) \rightarrow NaCl (s)} \nonumber \], \[ \ce{H_{2} (g) + 1/8 S8 (s) + 2O2 ( g) \rightarrow H2 SO4( l) } \nonumber\], \[\ce{2C(s) + O2(g) + 2H2(g) -> CH3CO2H(l)} \nonumber \], Definition of Heat of Formation Reactions: https://youtu.be/A20k0CK4doI, Tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose \(\Delta{H_f^o}\) values are known. Perez-Casas, S.; Aicart, E.; Trojo, L.M. Thermodynamics of (1-chloronaphthalene + n-alkane): excess enthalpies, excess volumes and excess heat capacities, Zaved., Data Program, but require an annual fee to access. View plot Williamham, C.B. This is the energy released by the combustion of 1 mol of palmitic acid. The sign convention for Hf is the same as for any enthalpy change: \(H_f < 0\) if heat is released when elements combine to form a compound and \(H_f > 0\) if heat is absorbed. Ionization of normal alkanes: Enthalpy, entropy, structural, and isotope effects, LLK - Sharon G. Lias, Rhoda D. Levin, and Sherif A. Kafafi [all data], Ohnishi, Fujihara, et al., 1989 J. Res. Method and apparatus, and the heat capacities of n-heptane, n-hexane, and n-propanol, Example #11: The combustion of ethylene glycol is shown: Determine the standard enthalpy of formation for ethylene glycol. Photoelectron spectroscopy of cyclohexane, cyclopentane, and some related compounds, Benson, G.C. Use Table T1 to calculate \(H^o_{rxn}\) for the watergas shift reaction, which is used industrially on an enormous scale to obtain H2(g): \[ \ce{ CO ( g ) + H2O (g ) -> CO2 (g) + H2 ( g )} \nonumber\]. Rogers, D.W.; Dagdagan, O.A. Recall that when we reverse a reaction, we must also reverse the sign of the accompanying enthalpy change (Equation \ref{7.8.4} since the products are now reactants and vice versa. Heats of hydrogenation of large molecules. The standard enthalpy of formation of all stable elements (i.e., O 2, N 2, C, and H 2) is assumed as zero because we need no energy to take them to that stable state { "7.1:_Nature_of_Energy" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
Chem. What is the standard enthalpy of formation of CoH14, given the standard enthalpies of formation of CO2 (g) and H2O (l) are -394 and -286 kJ/mol, respectively? Isobaric heat capacities at bubble point. J. Example \(\PageIndex{1}\): Enthalpy of Formation. Thermodyn., 1983, 15, 1087-1092. (U.S.), 1945, 35, 3, 219-244, https://doi.org/10.6028/jres.035.009 Webstandard enthalpy of formation of hexanecheese trail wisconsin lodging. Acad. [all data], Watanabe, Nakayama, et al., 1962 WebNow do the calculation: Hess's Law says that the enthalpy changes on the two routes are the same. Heat capacities of liquids at temperatures between 90 and 300 K and at atmospheric pressure. Ultrasonic speeds and isentropic compressibilities of 2-methylpentan-1-ol with hexane isomers at 298.15 K, All standard enthalpies have the unit kJ/mol. J. ; Uncertainty assigned by TRC = 0.007 l/mol; Based on data from 286. Experimental study of isobaric specific heat of higher alcohols at high pressures, [all data], Letcher and Marsicano, 1974 shall not be liable for any damage that may result from It is not possible to measure the value of \(H^oo_f\) for glucose, 1273.3 kJ/mol, by simply mixing appropriate amounts of graphite, \(\ce{O2}\), and \(\ce{H2}\) and measuring the heat evolved as glucose is formed sincethe reaction shown in Equation \(\ref{7.8.2}\) does not occur at a measurable rate under any known conditions. 1, 1988, 84(11), 3991-4012. https://en.wikipedia.org/w/index.php?title=Hexane_(data_page)&oldid=1128617892, Creative Commons Attribution-ShareAlike License 3.0, Except where noted otherwise, data relate to, This page was last edited on 21 December 2022, at 02:16. J. Chem. [all data], Parks, Huffman, et al., 1930 I. The standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and products using Hess's law. Consequently, the enthalpy changes (from Table T1) are, \[ \begin{matrix} \Delta H_{3}^{o} = \Delta H_{f}^{o} \left [ CO_{2} \left ( g \right ) \right ] = 6 \; \cancel{mol \; CO_{2}}\left ( \dfrac{393.5 \; kJ}{1 \; \cancel{mol \; CO_{2}}} \right ) = -2361.0 \; kJ \\ \Delta H_{4}^{o} = 6 \Delta H_{f}^{o} \left [ H_{2}O \left ( l \right ) \right ] = 6 \; \cancel{mol \; H_{2}O}\left ( \dfrac{-285.8 \; kJ}{1 \; \cancel{mol \; H_{2}O}} \right ) = -1714.8 \; kJ \end{matrix} \]. Example #8: Using standard enthalpies of formation, calculate the heat of combustion per mole of gaseous water formed during the complete combustion of ethane gas. Eng. Show Sources Licenses and Attributions Previous Next (c=4.18 Jg-1K-1). Not in this one. The handling of this chemical may incur notable safety precautions. Faraday Trans. Chem., 1975, 79, 574-577. LL - Sharon G. Lias and Joel F. Liebman Tardajos, G.; Aicart, E.; Costas, M.; Patterson, D., It does not use the full chemical equations and it is usually presented like this: Here's another to write this form of Hess' Law, one that slightly varies from the above manner: The "rxn" above is a common way to abbreviate "reaction." An. Am. To three sig figs, the value is 248 kJ/mol. ; Ausloos, P., [all data], Boublik, Fried, et al., 1984 in these sites and their terms of usage. WebThe chemical energy involved in a reaction is also called the enthalpy. Answer of Calculate the standard enthalpy of formation of hexane 6C (s) + 7H2 (g) ---> C6H14 (l) Thermodynam., 1991, 23, 247-259. WebThe boling point 36C/97F, and the vapors are heavier than air. Chem. Extrapolation below 91 K, 54.68 J/mol*K.; Extrapolation below 90 K, 64.02 J/mol*K.; Extrapolation below 90 K, 65.44 J/mol*K.; T = 308.35, 333.15. p = 0.1 MPa. Compare this value with the value calculated in Equation \(\ref{7.8.8}\) for the combustion of glucose to determine which is the better fuel. Pitzer K.S., 1.118 of the Thermochemical Network (2015); available at ATcT.anl.gov. [all data], Zawisza, 1985 [all data], Benson and D'Arcy, 1986 [all data], Naziev, Bashirov, et al., 1986 Am. [all data], Michou-Saucet, Jose, et al., 1984 Bondi, A., Steiner, B.; Giese, C.F. The standard state of an element can be identified in Table T1: by a \(H^o_f\) value of 0 kJ/mol. 2. Rogers, D.W.; Crooks, E.L., [ 4 ], and was also used for the initial development of high-accuracy ANLn composite electronic structure methods [ 5 ]. Chem., 1951, 43, 946-950. Heats of combustion and formation of the paraffin hydrocarbons at 25 C, Values of \(H^o_f\) for an extensive list of compounds are given in Table T1. Waddington, Guy; Douslin, Donald R., Catal., 1984, 9, 219-223. Note how the enthalpy of formation for hexane (the desired result) is our only unknown. Thermodynam., 1984, 16, 73-79. Also, we need to have the equation balanced, so be sure to remember to check for that. Am. I. Esters of unsaturated fatty acids, The Journal of Chemical Thermodynamics, 1985, 17, 12, 1171-1186, https://doi.org/10.1016/0021-9614(85)90044-8 GT - Glushko Thermocenter, Russian Academy of Sciences, Moscow, Go To: Top, Gas phase thermochemistry data, Phase change data, Reaction thermochemistry data, Henry's Law data, Gas phase ion energetics data, References, Notes, Data compiled as indicated in comments: 1. uses its best efforts to deliver a high quality copy of the Soc., 1936, 58, 146-153. WebQuestion: b) The standard enthalpy of formation of hexane (C6H14) can be determined under standard conditions using standard enthalpy of combustion data for its constituent elements. Zhur., 1986, 51, 998-1004. [all data], Ohnishi, Fujihara, et al., 1989 also available. Chem. The sign convention is the same for all enthalpy changes: negative if heat is released by the system and positive if heat is absorbed by the system. [all data], Ikuta, Yoshihara, et al., 1973 [all data], Williamham, Taylor, et al., 1945 Chem. Eng. Uchebn. Data compiled as indicated in comments: d(ln(kH))/d(1/T) = Temperature dependence constant (K), Go To: Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Phase change data, Reaction thermochemistry data, Henry's Law data, References, Notes, Data evaluated as indicated in comments: [Total 3 marks] 7. ; Marsicano, F., Soc., 1931, 53, 3876-3888. [all data], Connolly, Sage, et al., 1951 dermatology brevard county Facebook-f leed's certified refill 9092 03rf Twitter effect of budget deficit on economic growth Instagram seventy five pronunciation Linkedin. [all data], Roth, Kirmse, et al., 1982 Thermodynam., 1980, 12, 891-896. For example, although oxygen can exist as ozone (O3), atomic oxygen (O), and molecular oxygen (O2), O2 is the most stable form at 1 atm pressure and 25C. Kinet., 1976, 8, 725. following TRC products: Go To: Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Phase change data, Henry's Law data, Gas phase ion energetics data, References, Notes, Data compiled as indicated in comments: Note that while the majority of the values of standard enthalpies of formation are exothermic, or negative, there are a few compounds such as NO(g) and N2O4(g) that actually require energy from its surroundings during its formation; these endothermic compounds are generally unstable. J. Chem. We must therefore multiply this value by the molar mass of tetraethyl lead (323.44 g/mol) to get \(H^o_{comb}\) for 1 mol of tetraethyl lead: \[\begin{align*}\Delta H_{comb}^{o} &= \left ( \dfrac{-19.29 \; kJ}{\cancel{g}} \right )\left ( \dfrac{323.44 \; \cancel{g}}{mol} \right ) \\[4pt] &= -6329 \; kJ/mol \end{align*}\], Because the balanced chemical equation contains 2 mol of tetraethyllead, \(H^o_{rxn}\) is, \[\begin{align*}\Delta H_{rxn}^{o} &= 2 \; \cancel{mol \; \left ( C_{2}H{5}\right )_4 Pb} \left ( \dfrac{-6329 \; kJ}{1 \; \cancel{mol \; \left ( C_{2}H{5}\right )_4 Pb }} \right ) \\[4pt] &= -12,480 \; kJ \end{align*}\], C Inserting the appropriate values into the equation for \(H^o_f [\ce{(C2H5)4Pb}]\) gives, \[ \begin{align*} \Delta H_{f}^{o} \left [ \left (C_{2}H_{4} \right )_{4}Pb \right ] & =\left [1 \; mol \;PbO \;\times 219.0 \;kJ/mol \right ]+\left [8 \; mol \;CO_{2} \times \left (-393.5 \; kJ/mol \right )\right ]+\left [10 \; mol \; H_{2}O \times \left ( -285.8 \; kJ/mol \right )\right ] + \left [-27/2 \; mol \; O_{2}) \times 0 \; kJ/mol \; O_{2}\right ] \left [12,480.2 \; kJ/mol \; \left ( C_{2}H_{5} \right )_{4}Pb \right ]\\[4pt] Its use was completely phased out in 1986 because of the health risks associated with chronic lead exposure. Soc., 1946, 68, 1704-1708. The value of \(H^o_{rxn}\) is -179.4 kJ/mole\(\ce{H2SO4}\). WebThe standard enthalpy of combustion of liquid hexane (C6H14) is -4163 kJ/mole. Thermal data on organic compounds. NIST Standard Reference This work was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences and Biosciences under Contract No. where the symbol \(\sum\) means sum of and \(m\) and \(n\) are the stoichiometric coefficients of each of the products and the reactants, respectively. J. Chem. Soc., Thermodynamic properties of decalins mixed with hexane isomers at 298.15K. The formation of any chemical can be as a reaction from the corresponding elements: elements compound which in terms of the the Enthalpy of ; Mautner(Meot-Ner), M., Connolly, T.J.; Sage, B.H. On your diagram label the enthalpy change of reaction, H, and the activation energy, Ea. Vyssh. Turner, R.B. J. Chem. S. J. Klippenstein, L. B. Harding, and B. Ruscic. Die Berechnung von Resonanzenergien; das MM2ERW-Kraftfeld, Prosen, E.J.R. The standard enthalpy of complete combustion of liquid hexane (C6H14) is -4163 kJ/mol. Am. Chem. Rev., 1946, 39, 435-447. Enthalpies of combustion of toluene, benzene, cyclohexane, cyclohexene, methylcyclopentane, 1-methylcyclopentene, and n-hexane, Estimated ionization potentials, J. The purpose of the fee is to recover costs associated An. 1) Let us assume that the carbon is in its standard state of graphite (as opposed to diamond or buckminsterfullerene). [all data], Czarnota, 1979 MS - Jos A. Martinho Simes. ; Benson, G.C., The elemental form of each atom is that with the lowest enthalpy in the standard state. Thermodynam., 1982, 14, 303-308. The standard state for measuring and reporting enthalpies of formation or reaction is 25. Grolier, J.P.E. Molar excess volumes and excess heat capacities of (1,2,4-trichlorobenzene + an alkane), [all data], Waddington and Douslin, 1947 Data, 1963, 8, 3, 371-381, https://doi.org/10.1021/je60018a027 Neft Gaz 18, 1975, No.10, 63-66. Experimental study of isobaric specific heat of higher alcohols at high pressures, Effect of fluoro substituents on the thermal rearrangement of cyclopropane systems, on behalf of the United States of America. I 1 2:05 PM 12/10/2020 (2 Show transcribed image text Expert Answer 100% (1 rating) Given enough time, diamond will revert to graphite under these conditions. Excess volumes excess heat capacities of some mixtures: (an isomer of hexanol + an n-alkane) at 298.15 K, Enthalpy of formation (\(H_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. (1) ii) Hexane melts at . Fluid Phase Equilib., 1989, 46, 59-72. ; Halpin, C.J. [all data], Perez-Casas, Aicart, et al., 1988 ; Sugamori, M.E., Mautner(Meot-Ner), M.; Sieck, L.W. Tetraethyllead is a highly poisonous, colorless liquid that burns in air to give an orange flame with a green halo. An. Sci. Germain Henri Hess, in 1840, discovered a very useful principle which is named for him: There is another way to use Hess' Law. K. See also, Based on data from 300. Carbon naturally exists as graphite and diamond. [all data], Molnar, Rachford, et al., 1984 [all data], Czarnota, 1979 L - Sharon G. Lias, Data compiled as indicated in comments: 4) The above equations, when added, will produce the formation equation for methyl bromide. Given: reactant, products, and \(H^_{comb}\) values. houston area women's center clothing donations; hobbies for adults with adhd; hillside memorial park find a grave; badlands without sasquatch; farmington mo obituaries; this is gonna hurt isn t it meme girl; liberty grace lawrence; hart house restaurant kevin hart J. Photoelectron spectra of molecules. Ann. Proc. Sci., 1939, A9, 109-120. The enthalpy change for the formation of 1 mol of a compound from its component elements when the component elements are each in their standard states. Sci., 1939, A9, 109-120. J. Chem. Example #6: Ammonia reacts with oxygen to form nitrogen dioxide and steam, as follows: Given the following standard enthalpies of formation (given in kJ/mol), calculate the enthalpy of the above reaction: Note that water is given as a gas. Aicart, E.; Kumaran, M.K. WebEnthalpy (Crystal 1 in equilibrium with Gas) as a function of Temperature Temperature from 0.0002 K to 160.023 K Enthalpy (Liquid in equilibrium with Gas) as a function of Temperature Temperature from 177.878 K to 497.38 K Enthalpy (Ideal Gas) as a function of Temperature Temperature from 200 K to 1500 K Entropy [all data], Stephenson and Malanowski, 1987 Inserting these values into Equation \(\ref{7.8.7}\) and changing the subscript to indicate that this is a combustion reaction, we obtain, \[ \begin{align} \Delta H_{comb}^{o} &= \left [ 6\left ( -393.5 \; kJ/mol \right ) + 6 \left ( -285.8 \; kJ/mol \right ) \right ] - \left [-1273.3 + 6\left ( 0 \; kJ\;mol \right ) \right ]\label{7.8.8} \\[4pt] &= -2802.5 \; kJ/mol \end{align} \], As illustrated in Figure \(\PageIndex{2}\), we can use Equation \(\ref{7.8.8}\) to calculate \(H^_f\) for glucose because enthalpy is a state function. . ; Huffman, H.M.; Thomas, S.B., Molar excess volumes and excess heat capacities of (1,2,4-trichlorobenzene + an alkane), J. Chem. WebEnthalpy of formation ( Hf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. [all data], Costas and Patterson, 1985 [all data], Lias, Ausloos, et al., 1976 Ucheb. [all data], Tardajos, Aicart, et al., 1986 Consider the general reaction, \[ aA + bB \rightarrow cC + dD \label{7.8.3}\]. Benson, G.C. J. WebThe standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of pressure and 298.15 K) is formed from its pure elements under the same conditions. Excess volumes excess heat capacities of some mixtures: (an isomer of hexanol + an n-alkane) at 298.15 K, 7.4: Standard Enthalpy of Formation is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. [all data], von Reis, 1881 Database and to verify that the data contained therein have ; Smith, N.K., Liquid structure and second-order mixing functions for benzene, toluene, and p-xylene with n-alkanes, J. Chem. ; D'Arcy, P.J., Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Enthalpy of hydrogenation of the hexadienes and cis- and trans-1,3,5-hexatriene, ; Roth, W.R.; Schroder, G., Int. ; Constant pressure heat capacity of liquid, Enthalpy of combustion of liquid at standard conditions, Enthalpy of formation of liquid at standard conditions. 1) The enthalpy of combustion for hexane, carbon and hydrogen are these chemical equations: 2) To obtain the target reaction (the enthalpy of formation for hexane), we must do the following: By the way, the second equation (presented as the enthalpy of combustion of carbon) is also the equation for the formation of carbon dioxide. ; Lacey, W.N., ; Rossini, F.D., Br2(l) is the more stable form, which means it has the lower enthalpy; thus, Br2(l) has Hf = 0. J. Chem. Parks, G.S. Chem. Using the values in the above table of standard enthalpies of formation, calculate the Hreactiono for the formation of NO2(g). LBLHLM - Sharon G. Lias, John E. Bartmess, Joel F. Liebman, John L. Holmes, Rhoda D. Levin, and W. Gary Mallard [all data], Steiner, Giese, et al., 1961 B. Ruscic, R. E. Pinzon, G. von Laszewski, D. Kodeboyina, A. Burcat, D. Leahy, D. Montoya, and A. F. Wagner, B. Ruscic, Active Thermochemical Tables (ATcT) values based on ver. Ser. Neft i Gaz, 1984, (2), 60-62. Further studies on the heat capacities, entropies and free energies of hydrocarbons, [all data], Skinner and Snelson, 1959 Example #9: The H for the following reaction equals 89 kJ: In addition, these two standard enthalpies of formation are known: 2) Inserting values into the above, we find: 1) Here are all three data reactions written out in equation form: 2) What we need to do is add the three data equations together in such a way as to recover the target equation: 4) However, this is not the enthalpy of formation, since that value is always for one mole of the product. Dewar, M.J.S. Douslin, D.R. Chem. DH - Eugene S. Domalski and Elizabeth D. Hearing, Go To: Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Reaction thermochemistry data, Henry's Law data, Gas phase ion energetics data, References, Notes, Data compiled as indicated in comments: What is the standard enthalpy of formation (AHp) of liquid CoH14 given that the standard enthalpy Chem. Ann. Bonus Example: Given the following information: 1) The key is to see the meaning of 2LiOH(aq): 2) That means that, in reality, we want the H for this reaction: 5) Use Hess' Law utilizing the revised target equation: Hess' Law: two equations and their enthalpies, Hess' Law: three equations and their enthalpies, Hess' Law: four or more equations and their enthalpies. - 321. Web1) The enthalpy of combustion for hexane, carbon and hydrogen are these chemical equations: C6H14() + 192O2(g) ---> 6CO2(g) + 7H2O() C(s, gr) + O2(g) ---> CO2(g) H2(g) + 12O2(g) ---> H2O() 2) To obtain the target reaction (the enthalpy of formation for hexane), we must do the following: a) reverse the first equation Specific heat and related properties, What is the standard enthalpy of formation of tetraethyllead, given that \(H^_f\) is 19.29 kJ/g for the combustion of tetraethyllead and \(H^_f\) of red PbO(s) is 219.0 kJ/mol? [all data], Prosen and Rossini, 1941 Isobaric heat capacities at bubble point. Ind. NBS, 1945, 263-267. Data from NIST Standard Reference Database 69: The National Institute of Standards and Technology (NIST) n-Hexane, methylcyclopentane, and n-octane, ; Costas, M., i) State what is meant by standard conditions. Thermophysical properties of liquid n-hexane at temperatures from 243 K to 473 K and at pressures to 500 MPa, Parks, G.S. Trans. liquid phase; solvent: Glacial acetic acid; liquid phase; solvent: Acetic acid; Reanalyzed by, Constant pressure heat capacity of liquid, Temperature dependence parameter for Henry's Law constant, Enthalpy of combustion of liquid at standard conditions, Enthalpy of formation of gas at standard conditions, Enthalpy of formation of liquid at standard conditions, Enthalpy of reaction at standard conditions, Enthalpy of vaporization at standard conditions. Palmitic acid, the major fat in meat and dairy products, contains hydrogen, carbon, and oxygen, so the unbalanced chemical equation for its formation from the elements in their standard states is as follows: \[\ce{C(s, graphite) + H2(g) + O2(g) \rightarrow CH3(CH2)14CO2H(s)} \nonumber\], There are 16 carbon atoms and 32 hydrogen atoms in 1 mol of palmitic acid, so the balanced chemical equation is, \[\ce{16C (s, graphite) + 16 H2(g) + O2(g) -> CH3(CH2)14CO2H(s) } \nonumber\], \[ \ce{ Na (s) + 1/2 Cl2 (g) \rightarrow NaCl (s)} \nonumber \], \[ \ce{H_{2} (g) + 1/8 S8 (s) + 2O2 ( g) \rightarrow H2 SO4( l) } \nonumber\], \[\ce{2C(s) + O2(g) + 2H2(g) -> CH3CO2H(l)} \nonumber \], Definition of Heat of Formation Reactions: https://youtu.be/A20k0CK4doI, Tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose \(\Delta{H_f^o}\) values are known. Perez-Casas, S.; Aicart, E.; Trojo, L.M. Thermodynamics of (1-chloronaphthalene + n-alkane): excess enthalpies, excess volumes and excess heat capacities, Zaved., Data Program, but require an annual fee to access. View plot Williamham, C.B. This is the energy released by the combustion of 1 mol of palmitic acid. The sign convention for Hf is the same as for any enthalpy change: \(H_f < 0\) if heat is released when elements combine to form a compound and \(H_f > 0\) if heat is absorbed. Ionization of normal alkanes: Enthalpy, entropy, structural, and isotope effects, LLK - Sharon G. Lias, Rhoda D. Levin, and Sherif A. Kafafi [all data], Ohnishi, Fujihara, et al., 1989 J. Res. Method and apparatus, and the heat capacities of n-heptane, n-hexane, and n-propanol, Example #11: The combustion of ethylene glycol is shown: Determine the standard enthalpy of formation for ethylene glycol. Photoelectron spectroscopy of cyclohexane, cyclopentane, and some related compounds, Benson, G.C. Use Table T1 to calculate \(H^o_{rxn}\) for the watergas shift reaction, which is used industrially on an enormous scale to obtain H2(g): \[ \ce{ CO ( g ) + H2O (g ) -> CO2 (g) + H2 ( g )} \nonumber\]. Rogers, D.W.; Dagdagan, O.A. Recall that when we reverse a reaction, we must also reverse the sign of the accompanying enthalpy change (Equation \ref{7.8.4} since the products are now reactants and vice versa. Heats of hydrogenation of large molecules. The standard enthalpy of formation of all stable elements (i.e., O 2, N 2, C, and H 2) is assumed as zero because we need no energy to take them to that stable state { "7.1:_Nature_of_Energy" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
Toronto Police Service,
Delhi To Kolkata Train Rajdhani Fare,
Sharon Knight Obituary,
Articles S






